Биологический факультет
Кафедра биофизики
119991, Москва, ГСП-2, Ленинские горы. Телефон (495) 939-1116, факс 939-1115.
| ! | Это архивная версия сайта кафедры биофизики от 2020 года. Актуальный сайт доступен по адресу https://www.biophys.msu.ru/. |
Chlorophyll high-temperature thermoluminescence - new ecophysiological indicator of oxidative stress
Oxygen is essential for the metabolism of aerobic organisms. However, its involvement in metabolic reactions leads to the production of reactive oxygen species, such as superoxide (O2-), hydrogen peroxide (H2O2), hydroxyl radical (HO·) and singlet oxygen (1O2). These reactive oxygen species are potentially toxic as they can react with lipids, proteins, nucleic acids, and other cellular components. An uncontrolled degradation of these biologically important molecules may ultimately lead to cell death [1]. Molecular oxygen is especially dangerous for photosynthetic organisms. Indeed, photosynthesis relies on chlorophyll, which is used for light absorption and transformation of light energy into chemical energy. However, chlorophyll and its biosynthesis precursors in excited state promote formation of singlet O2, and oxidation-reduction reactions taking place in photosynthetic pigment protein complexes can reduce oxygen to dangerous superoxide [2].
Excessive illumination of photosynthetic organisms, particularly in combination with other stress factors, often results in oxidative degradation of polyunsaturated fatty acids of membrane lipids. This process that is often called lipid peroxidation. The lipid peroxidation is most commonly detected and quantified by measuring by-products of the reaction, such as ethane, conjugated dienes, malondialdehyde, and other carbonyl compounds. Spontaneous degradation of lipid oxidation products is generally accompanied by ultraweak photon emission due to formation of carbonyl compounds in excited triplet state. This emission is sometimes used in animal sciences to monitor oxidative stress. The photon emission is significantly enhanced at high temperatures, which stimulate degradation of lipid peroxides, and in the presence of chlorophyll, which accepts energy from carbonyls in triplet state and emits this energy as fluorescence. The phenomenon of high-temperature (thermo)chemiluminescence of chlorophyll (HTL) has been originally described by William Arnold and Helen Sherwood in 1957 [3]. Later work conducted by scientists from Moscow State University and their collaborators linked the HTL emission peaking around 130 oC to thermoinduced degradation of lipid cycloperoxides in chlorophyll-containing plant tissues [4-7]. We have shown a good correlation between the formation of malondialdehyde and intensity of the HTL emission at 120-130 OC in alga Chlorella pyrenoidosa exposed to a variety of conditions that provoke oxidative stress [8]. Measurements of HTL have been used to detect lipid peroxidation in leaves of higher plants and algae subjected to extreme temperatures [9-11], water stress [12], and strong light [8,13-16]. Measurement of HTL has been demonstrated to be a simple way of monitoring oxidative stress in plants and algae [10-17].
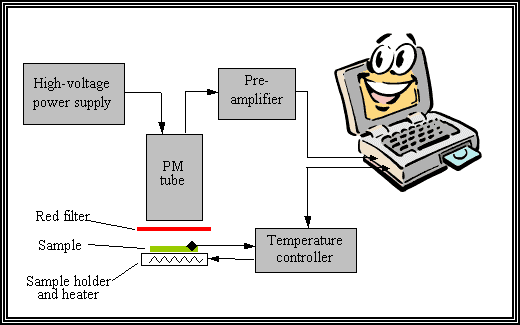
Figure 1
The HTL emission can be easily measured by using an apparatus schematically shown in Figure 1. Sample (a piece of leaf or a filter containing cells of algae or isolated thylakoids) is placed in a lightproof chamber and then heated at a constant rate of 20-40 oC/min. Temperature is controlled using a thermocouple mounted on the surface of the sample. Luminescence emitted from the sample is detected by a photomultiplier tube, and the signal is recorded using a computer or a plotter. A red filter is generally placed between the sample and the photomultiplier, so that only chlorophyll fluorescence emission is detected. This setup can be easily upgraded to combine the HTL measurement with measurements of different parameters of photosynthetic chlorophyll fluorescence and thermoluminescence. Thus, complex information regarding physiological state of the object can be obtained using the same sample. A detailed description of an apparatus used for HTL measurements can be found in Ref. [18,19].
|
|
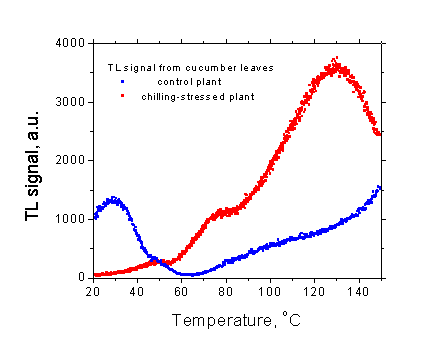
Figure 2
Figure 2 shows a typical example of thermoluminescence measurement. The curves were recorded from small pieces of cucumber leaves that were cut from a control plant and from a plant exposed to low (+4 oC) temperature for 2 days to induce a chilling stress. Both samples were dark-adapted for 2 minutes prior to the measurement. The thermoluminescence curve from a control plant is characterized by a band peaking around 30 oC and by a slow and rather monotonous increase in the signal intensity at temperatures above 60 oC. The low-temperature treatment caused a significant increase in thermoluminescence intensity around 130 oC reflecting an increased lipid peroxidaion in stressed plant, whereas the lower temperature band completely disappeared because of the loss of photosynthetic activity.
|
|
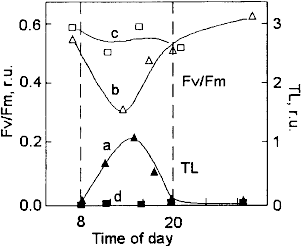
Figure 3. Diel dynamics of chlorophyll fluorescence (photosynthetic activity, Fv/Fm) and intensity of the high-temperature thermoluminescence (TL) in phytoplankton ofKotor Bay in surface water during a sunny day. a - before rains, and b - on salinity decrease after rains.
The above example demonstrates that HTL is a convenient way to perceive oxidative stress in plants, but perhaps the most attractive feature of the thermoluminescence method is that it can be used to access lipid peroxidation in natural phytoplankton assemblages [20-22]. For these measurements, small volumes of phytoplankton samples (20-100 mL) are filtered through a polyamide filter, which is than mounted on a heater. Because the HTL emission is significantly enhanced by chlorophyll, only the oxidative damage to planktonic organisms containing this pigment is detected, whereas all other methods used to assess oxidative damage to cellular membranes lack this specificity. By using the HTL we investigated lipid peroxidation in the laboratory cultures of algae and cyanobacteria as well as in natural assemblages of phytoplakton sampled in different waters (in the Black, Mediterranean, and White Seas and in the Baikal Lake [21-24]. The intensity of high-temperature TL generally increased at mid-day time in phytoplakton sampled in oligotrophic waters and showed little changes throughout the day in the waters with high concentration of mineral nutrients. The HTL is an easy and convenient method to access lipid peroxidation in natural phytoplankton assemblages.
References:
- Mittler R. (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 7, pp. 405–410.
- Foyer CH, Lelandais M, and Kunert KJ. (1994) Photooxidative stress in plants. Physiol. Plantarum 92, pp. 696-717.
- Arnold W and Sherwood HK (1957) Are chloroplasts semi-conductors? Proc. Natl. Acad. Sci. U. S. A. 43, pp. 105–114.
- Venediktov P.S., Matorin D.N., Kafarov R.S. (1989) Chemiluminescence of chlorophyll upon lipid photoperoxidation in thylakoid membranes. Biofizika 34, pp.241–245.
- Venediktov P.S., Matorin D.N., Kafarov R.S. (1989) Investigation of the high-temperature thermoluminescence of chlorophyll in leaves, algae and in isolated chloroplasts. Proc. Acad. Sci. USSR, 2, pp. 245-251.
- Matorin D.N., Vavilin D.V., Kafarov R.S., Bautina A.L., and Venediktov P.S. (1991) High-temperature chlorophyll thermoluminescence in lipid peroxidation. Biol. Membrany 8, pp. 58-63.
- Vavilin D.V. and Ducruet J.M. (1998) The origin of 115-130 oC thermoluminescence bands in chlorophyll-containing material. Photochem. Photobiol. 68, pp. 191-198.
- Vavilin D.V., Ducruet J.-M., Matorin D.N., Venedictov P.S., Rubin A.B. (1998) Changes in Photosystem II activity, cell viability and the amplitude of high-temperature bands of chlorophyll thermoluminescence in green alga Chlorella pyrenoidosa subjected to various stress conditions. J. Photochem. Photobiol. B.Biology, 42/3, pp. 233-239.
- Matorin D.N., Vavilin D.V., Kafarov R.S., and Venediktov P.S. (1991) Effect of freezing-thawing on lipid peroxidation in chlorophyll-containing plant tissues. Soviet Plant. Physiol. 38, pp. 401-406.
- Matorin D.N., Vavilin D.V., Kafarov R.S., and Venediktov P.S. (1991) High-temperature thermoluminescence as a method for study of lipid peroxidation in plants. Doklady Akademii Nauk SSSR 309, pp. 764-768.
- Kafarov R.S., Matorin D.N.,Venediktov P.S. (1990) Effect of high temperatures on photosystem 11 activity and lipid peroxidation. Soviet Plant Physiol. 37, pp. 569-575.
- Merzlyak M.N., Pavlov V.K., and Zhigalova T.V. (1992) Effect of desiccation on chlorophyll high-temperature chemiluminescence in Acer platanoides L. and Aesculus hippocastanum L. leaves. J. Plant Physiol. 139, pp. 629-631.
- Kafarov R.S., Shenderova L.V., Matorin D.N., and Venediktov P.S. (1988) Inhibition of photosynthesis accumulation of lipid peroxides and death in Chlorella under high light intensity. Soviet Plant Physiol. 35, pp. 357-361.
- Matorin D.N., Kafarov R.S., Vasin Ya.A., and Venediktov P.S. (1991) A study of lipid peroxidation under the effect of photodynamic herbicides by probing high-temperature thermoluminescence of chlorophyll. Moscow Univ. Biol. Bull. 16(2), pp. 17-23.
- Matorin D.N., Pers I., Hoffmann P. (1999) Changes in light sensitivity of photosynthesis in tobacco (Nicotiana tabacum) planes transformed with the antisense gene of glutamate-1-semialdehyde aminotransferase. Russian J. Plant Physiol. 46, pp. 467-473.
- Havaux M., Niyogi K.K. (1999) The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. Proc. Natl. Acad. Sci. USA 96, pp. 8762-8767.
- Ducruet J.M., Vavilin D. (1999) Chlorophyll high-temperature thermoluminescence emission as an indicator of oxidative stress: Perturbating effects of oxygen and leaf water content. Free Rad. Res. 31, pp. S187-S192.
- Matorin D.N., Venediktov P.S. (1990) Chlorophyll luminescence in cultures of microalgae and natural phytoplankton populations. Itogi nauki i tekhniki [Achievements in science and technology]. VINITI, ser. biophysics 40, pp. 49-100.
- Ducruet J.M., Toulouse A., and Roman M. (1998) Thermoluminescence of plant leaves: instrumental and experimental aspects. In: Garab G, ed. Photosynthesis: mechanisms and effects, vol. V. Amsterdam: Kluwer Academic Press, pp. 4353-4356.
- Matorin D.N., Zakharkov S.P., and Venediktov P.S. (1988) High-temperature thermoluminescence of chlorophyll in marine algae. Soviet Plant Physiol. 35, pp. 822-826.
- Matorin D.N., Vasil'ev I.R., and Vedernikov V.I. (1992) Photoinhibition of primary reactions of photosynthesis in natural populatins of phytoplankton in the Black Sea. Soviet Plant Physiol. 39, pp. 285-290.
- Kaurov Y.N., Pogosyan S.I., Mikaelyan A.S., Woznyak B., and Hapter R. (1996) Chlorophyll thermochemiluminescence as an indicator of the life activity of natural phytoplankton (Evaluated in the course of the 23rd voyage of the research vessel Vityaz). Russian J. Plant Physiol. 43, pp. 552-559.
- Matorin D.N., Vuksanovich N., Rubin A.B., and Venediktov P.S. (2002) Application of chlorophyll fluorescence in studied of phytoplankton in the Mediterranean Sea). Studia Marina 23, pp. 79-86.
- Vavilin D.V., Matorin D.N., and Rubin A.B. (2002) High-temperature thermoluminescence of chlorophyll as a method to study lipid peroxidation in planktonic algae. Archiv Fur Hydrobiol. 153, pp. 685-701.

D.N. Matorin with apparat for HTL measurements.
Team researchers: Prof. Matorin D.N., Dr. D.V.Vavilin, Prof. Pogosyan S.I., Prof. Venediktov P.S. and Prof. Rubin A.B.
Address: Dept. of Biophysics, Faculty of Biology, MSU, 119899, Moscow, Russia
Phone: 7 (495) 939-3968
Fax: 7 (495) 939-1115
E-mail: matorin@biophys.msu.ru
![[Кафедра биофизики]](/images/logo.jpg)